×
The Standard e-Paper
Truth Without Fear
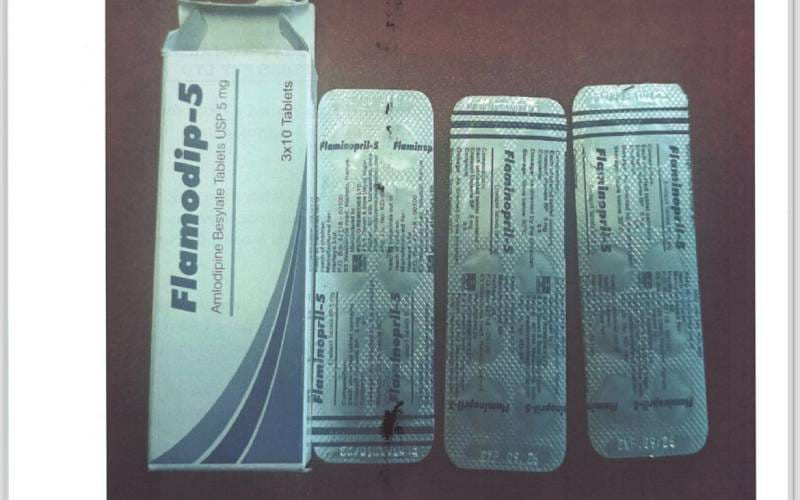
Due to a labeling error, the Pharmacy and Poisons Board (PPB) has recalled Flamodip tablets, manufactured by Medico Remedies.
In a statement, the Board noted that the product's labeling does not accurately reflect its content.